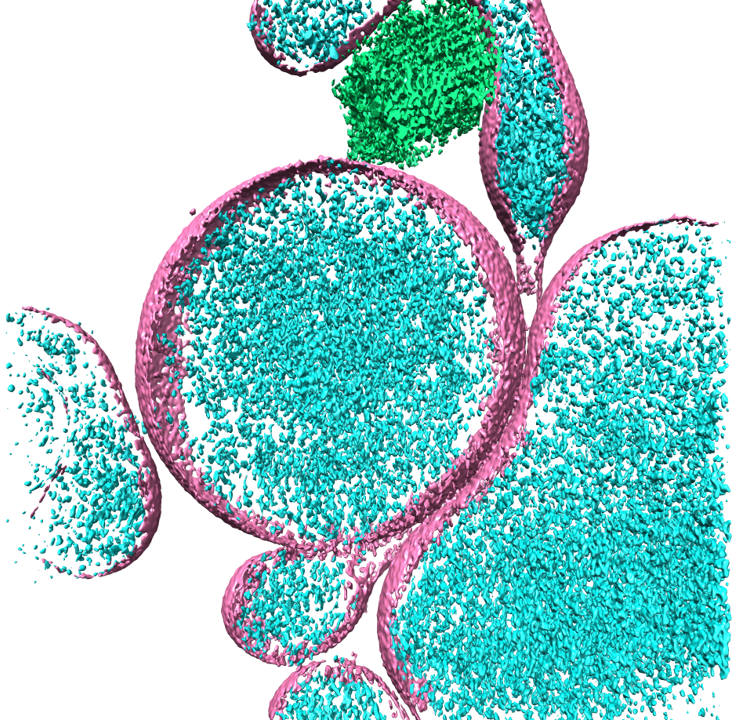
 3D reconstruction of cryo-ET tomogram showing huntingtin inclusions (blue) inside extracellular vesicles (pink).
|
Huntington's DiseaseIntrinsically disordered proteins (IDPs) make up about 40% of proteins in eukaryotic cells and play many important roles in cellular processes and signaling pathways. In fact, many proteins associated with neurodegenerative diseases are IDPs, that exhibit "prion-like" behaviors, in which they are transmitted from cell-to-cell, spreading pathological proteins that act as nucleation sites for aggregates. The goal of this research is to seek answers on how neurodegenerative IDPs spread, using mutant huntingtin protein (HTT), associated with Huntington's Disease, as a model. Evidence shows that when mutant HTT's poly-glutamine (polyQ) track is expanded, the protein has a higher propensity to aggregate and is more transmissive in nature. Since membranes modulate the formation of IDP inclusions, protein-membrane interactions may be promoting transmission. We take an interdisciplinary approach by combining cryo-ET with biochemical and biophysical assays to elucidate the molecular mechanisms of HTT transmission and to understand how interactions with membranes affect mutant HTT aggregation and potential spread. We hope to shed light on fundamental characteristics that influence neurodegeneration, opening new avenues for more precise and effective therapeutic approaches.
|
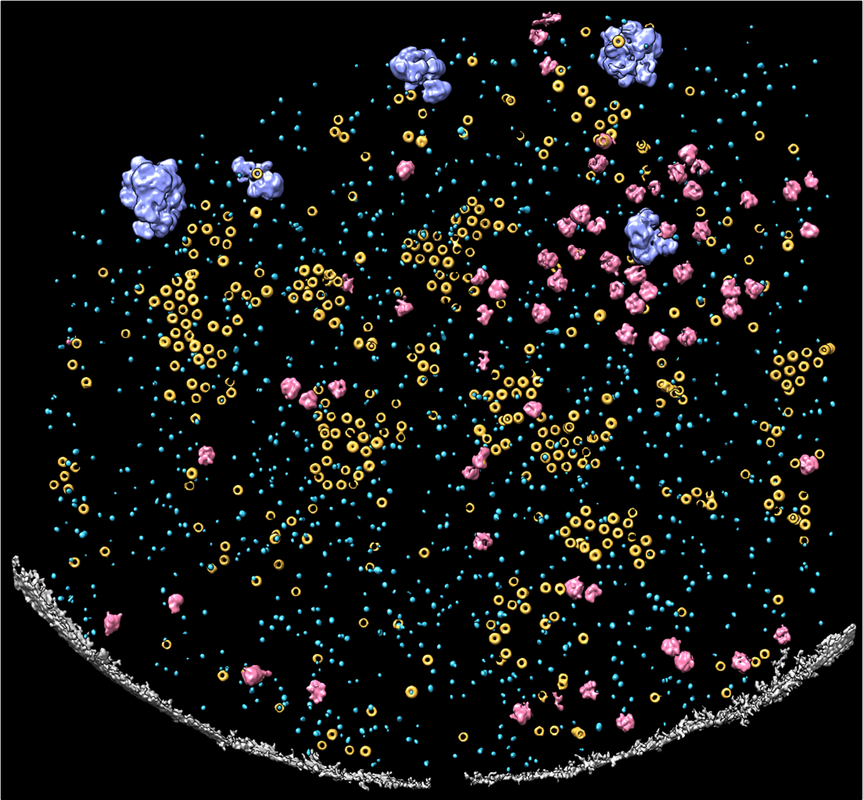 Annotation of various structural features in a C. glabrata plasma membrane tomogram showing Pma1 hexamers (yellow), GS (light blue), ribosomes (pink), and glycogen granules (purple) in the context of the plasma membrane (gray).
|
Antifungal Drug ResistanceInvasive fungal infections have become a significant global health concern, affecting over a billion people and causing approximately 3.75 million deaths worldwide each year. The rise of antifungal resistance further underscores the urgent need for next-generation therapeutic agents to effectively combat this emerging threat.
The fungal plasma membrane and its resident membrane proteins are promising antifungal drug targets. One key membrane protein, β-(1,3)-glucan synthase (GS), is the molecular target of the FDA-approved echinocandin drug class. GS is a membrane-embedded protein complex responsible for synthesizing glucans, a core structural component of the fungal cell wall. Despite decades of biomedical research and the availability of high-resolution structures of detergent-purified GS, the molecular mechanisms underlying echinocandin inhibition of GS function remain elusive. Our research aims to characterize the spatial distribution and structural dynamics of GS within its native membrane environment. Our preliminary studies suggest that the structure and functional dynamics of GS are interconnected with its native plasma membrane environment. With growing evidence indicating that membrane lipids may play a role in mediating GS-echinocandin interactions, we will integrate multimodal bioimaging tools with functional and biophysical analyses to unravel the functional implications of the plasma membrane in echinocandin action. Our work will enhance our understanding of GS as a critical enzyme in cell wall synthesis and a highly acclaimed drug target, providing much-needed insights to tackle the growing challenge of antifungal drug resistance. |